Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
| 1. | Which
statement concerning relative rates of reaction is correct for the decomposition of
phosphine?
4 PH3(g) ®
P4(g) + 6 H2(g)
a. | The rate of disappearance of PH3 is 6/4 the rate of
appearance of H2. | b. | The rate of appearance of P4 is equal to the rate of
appearance of H2. | c. | The rate of disappearance of PH3 is 1/4 the rate of
appearance of P4. | d. | The rate of disappearance of PH3 is 4 times the rate
of appearance of H2. | e. | The rate of appearance of H2 is 6 times the rate of
appearance of P4. | | |
|
|
| 2. | Which
relationship is correct for the rate of decomposition of dinitrogen
pentaoxide?
2 N2O5(g) ® 4
NO2(g) + O2(g)
|
|
| 3. | Ozone
decomposes to oxygen according to the balanced chemical equation below.
2 O3(g) ® 3 O2(g)
If the rate of disappearance of ozone is -7.2 ´ 10-4
M/s, what is the rate of formation of oxygen? a. | 4.8 ´ 10-4 M/s | b. | 7.2 ´ 10-4
M/s | c. | 1.1 ´ 10-3
M/s | d. | 1.4 ´ 10-3
M/s | e. | 2.2 ´ 10-3
M/s | | |
|
|
| 4. | Which
of the following changes are likely to increase the rate of a chemical
reaction?
1. | Adding a catalyst | 2. | Increasing the temperature of the
reactants | 3. | Increasing the
concentrations of the reactants | | |
a. | 1
only | b. | 2
only | c. | 3
only | d. | 1 and
3 | e. | 1, 2, and
3 | | |
|
|
| 5. | What
is the name given to a substance that increases the rate of a chemical reaction but is not itself
consumed? a. | reactant | b. | product | c. | intermediate | d. | catalyst | e. | element | | |
|
|
| 6. | What
is the overall order of the reaction below
2 NO(g) + 2 H2(g) ® N2(g) + 2
H2O(g)
if it proceeds via the following rate
expression?
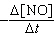 = k[NO]2[H2]
= k[NO]2[H2]a. | zero-order | b. | first-order | c. | second-order | d. | third-order | e. | fourth-order | | |
|
|
| 7. | Carbon monoxide reacts with oxygen according to the equation
below.
2 CO(g) + O2(g) ® 2
CO2(g)
What is the overall order of the
reaction? a. | first-order | b. | second-order | c. | third-order | d. | fourth-order | e. | not enough
information is given to determine the reaction order | | |
|
|
| 8. | Given
the initial rate data for the decomposition reaction,
A ® B + C
determine the rate expression for the
reaction.
[A], M | -D[A]/Dt M/s | 0.110 | 7.81
´
10-3 | 0.220 | 3.12
´
10-2 | 0.330 | 7.03
´
10-2 | | |
a. | 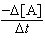 = 7.10 ´
10-2s-1[A] = 7.10 ´
10-2s-1[A] | b. | 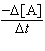 = 0.645 M-1s-1[A]2
= 0.645 M-1s-1[A]2 | c. | 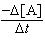 = 3.63 ´
10-2M-1s-1[A]2 = 3.63 ´
10-2M-1s-1[A]2 | d. | 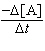 = 3.63 ´
10-2M s-1 = 3.63 ´
10-2M s-1 | e. | 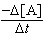 = 5.87 M-2s-1[A]3
= 5.87 M-2s-1[A]3 | | |
|
|
| 9. | Given
the initial rate data for the reaction A + B ® C, determine the rate expression for the
reaction.
[A], M | [B],
M | D[C]/Dt (initial) M/s | 0.250 | 0.150 | 8.90
´
10-6 | 0.250 | 0.300 | 1.78
´
10-5 | 0.500 | 0.300 | 7.12
´
10-5 | | | |
a. | 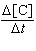 = 2.37 ´ 10-4
M-1s-1 [A][B]
= 2.37 ´ 10-4
M-1s-1 [A][B] | b. | 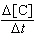 = 9.49 ´ 10-4
M-2s-1 [A]2[B]
= 9.49 ´ 10-4
M-2s-1 [A]2[B] | c. | 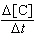 = 1.58 ´ 10-3
M-2s-1 [A][B]2 = 1.58 ´ 10-3
M-2s-1 [A][B]2 | d. | 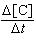 = 6.33 ´ 10-3
M-3s-1 [A]2[B]2 = 6.33 ´ 10-3
M-3s-1 [A]2[B]2 | e. | 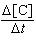 = 3.80 ´ 10-3
M-3s-1 [A]3[B] = 3.80 ´ 10-3
M-3s-1 [A]3[B] | | |
|
|
| 10. | For
the reaction A + 2B ® C, the rate law is
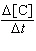 = k[A][B]2.
= k[A][B]2.
What are the
units of the rate constant where time is measured in seconds?
|
|
| 11. | For
the reaction 2A + B ® C, the rate law is
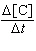 = k[A]2[B].
= k[A]2[B].
Which of the
factor(s) will affect the value of the rate constant for this
reaction?
1. | Increasing the concentration of reactant
A | 2. | Adding a
catalyst | 3. | Increasing the
temperature | | |
a. | 1
only | b. | 2
only | c. | 3
only | d. | 2 and
3 | e. | 1, 2, and
3 | | |
|
|
| 12. | The
reaction of NO and O2 produces NO2.
2 NO(g) + O2(g) ® 2 NO2(g)
The reaction is second-order with respect to NO(g) and first-order
with respect to O2(g). At a given temperature, the rate constant, k, equals 6.1
´ 103
M-2s-1. What is the rate of reaction when the initial concentrations of NO and
O2 are 0.020 M and 0.015 M, respectively? a. | 9.8 ´
10-10 M/s | b. | 0.027 M/s | c. | 0.037
M/s | d. | 1.0
M/s | e. | 1.8
M/s | | |
|
|
| 13. | How
are the exponents in a rate law determined?
1. | They are equal to the concentrations of the
reactants. | 2. | They are equal to the coefficients in the balanced chemical
equation. | 3. | They are
determined by experimentation. | | |
a. | 1
only | b. | 2
only | c. | 3
only | d. | 1 and
2 | e. | 2 and
3 | | |
|
|
| 14. | For a
second-order decomposition reaction,
2A ® B rate =
k[A]2
which of the
following functions can be plotted versus time to give a straight line? a. | [A] | b. | [A]2 | c. | ln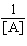 | d. | ln[A] | e. | | | |
|
|
| 15. | A
student analyzed a first-order reaction and obtained the graph below. Unfortunately, the student
forgot to label the axes. What are the correct labels for the x and y
axes?
a. | x axis = time, y
axis = ln[A] | b. | x axis = time, y axis = [A] | c. | x axis = time, y
axis = 1/[A] | d. | x axis = 1/Time, y axis = [A] | e. | x axis = 1/Time,
y axis = 1/[A] | | |
|
|
| 16. | Which
of the following equations corresponds to the integrated expression for a second-order decomposition
reaction? a. | [A] = -kt
+ [A]0 | b. | 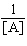 = kt +
= kt + 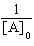 | c. | ln[A] =
-kt + ln[A]0 | d. | 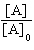 = -kt
= -kt | e. | 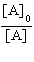 = -kt
= -kt | | |
|
|
| 17. | The
half-life of a first-order decomposition reaction is 2.36 hours. If the initial concentration of
reactant is 0.52 M, what is the concentration of reactant after 752 seconds? a. | 0.046
M | b. | 0.16
M | c. | 0.26
M | d. | 0.47
M | e. | 0.49
M | | |
|
|
| 18. | For
the first-order decomposition of N2O5 at 340 K, where k = 5.8
´ 10-3
s-1, calculate the original concentration if the concentration of
N2O5 is 0.41 M after 209 seconds. a. | 0.12
M | b. | 0.23
M | c. | 0.72
M | d. | 1.4
M | e. | 1.7
M | | |
|
|
| 19. | Which
of the following equations corresponds to the integrated expression for a zero-order decomposition
reaction? a. | [A] = -kt
+ [A]0 | b. | ln[A] = -kt +
ln[A]0 | c. | 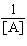 = kt +
= kt + 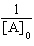 | d. | 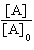 = -kt = -kt | e. | 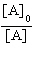 = -kt = -kt | | |
|
|
| 20. | The
reaction A ® B follows first-order kinetics with a half-life of 21.7 seconds. If
the concentration of A is 0.430 M after 14.5 seconds, what is the initial concentration of
A? a. | 0271
M | b. | 0.287
M | c. | 0.644
M | d. | 0.683
M | e. | 1.59
M | | |
|
|
| 21. | For
the zero-order reaction below, a graph of ____ versus time will generate a straight
line.
A ® B + C rate = k[A]0
|
|
| 22. | According to collision theory, which condition(s) must be met in order for molecules
to react?
1. | The reacting molecules must collide with each other to
react. | 2. | A catalyst must
be in contact with the reacting molecules for a reaction to occur. | 3. | The reacting molecules must collide with an orientation that
can lead to rearrangement of the atoms. | | |
a. | 1
only | b. | 2
only | c. | 3
only | d. | 1 and
2 | e. | 1 and
3 | | |
|
|
| 23. | In
general, as temperature increases, the rate of a chemical reaction a. | decreases due to
fewer collisions with proper molecular orientation. | b. | decreases for
endothermic reactions. | c. | decreases due to an increase in the activation
energy. | d. | increases due to a decrease in the activation
energy. | e. | increases due to a greater number of effective
collisions. | | |
|
|
| 24. | The
Arrhenius equation, k = A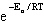 relates the rate of reaction and temperature. A plot of ln(k) versus 1/T
will yield a straight line with a slope of ____. relates the rate of reaction and temperature. A plot of ln(k) versus 1/T
will yield a straight line with a slope of ____.
|
|
| 25. | Molecules must overcome a barrier called the activation energy if they are to react.
The highest energy point reached during the progress of a reaction is called the
____. a. | transition
state | b. | Arrhenius state | c. | half-life | d. | elementary step | e. | intermediate
state | | |
|
|
| 26. | For a
chemical reaction, the activation energy for the forward reaction is +112 kJ and the activation
energy for the backward reaction is +187 kJ. What is the overall energy change for the forward
reaction? a. | -299
kJ | b. | -75
kJ | c. | -0.599
kJ | d. | +75
kJ | e. | +299
kJ | | |
|
|
| 27. | Calculate the activation energy, Ea for
N2O5(g) ® 2
NO2(g) + 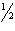 O2(g)
O2(g)
given k (at 25.0 °C) = 3.46 ´ 10-5
s-1 and k (at 45.0 °C) = 5.79 ´ 10-4 s-1. (R = 8.314
J/K·mol) a. | 1.32
kJ/mol | b. | 1.61 kJ/mol | c. | 14.0
kJ/mol | d. | 48.2 kJ/mol | e. | 111
kJ/mol | | |
|
|
| 28. | For a
given reaction, the rate constant doubles when the temperature is increased from 37 °C to 51
°C. What is the
activation energy for this reaction? (R = 8.314 J/K·mol) a. | 18
kJ/mol | b. | 41 kJ/mol | c. | 59
kJ/mol | d. | 86 kJ/mol | e. | 780
kJ/mol | | |
|
|
| 29. | The
effect of adding a catalyst to a reaction is to a. | increase the number of collisions between
reactants. | b. | increase the energy of the products. | c. | increase the
equilibrium constant of a reaction. | d. | lower the activation energy of a
reaction. | e. | decrease the enthalpy change of a
reaction. | | |
|
|
| 30. | The
elementary steps for the catalyzed decomposition of dinitrogen monoxide are shown
below.
N2O(g) + NO(g) ®
N2(g) + NO2(g)
2 NO2(g) ® 2 NO(g) +
O2(g)
Which of the following statements are
CORRECT?
1. | The overall balanced reaction is 2 N2O(g)
® 2
N2(g) + O2(g). | 2. | NO(g) is a catalyst for the reaction. | 3. | N2(g) is a reaction
intermediate. | | |
a. | 1
only | b. | 2
only | c. | 3
only | d. | 1 and
2 | e. | 1, 2, and
3 | | |
|
|
| 31. | In
basic solution, (CH3)3CCl reacts according to the equation
below.
(CH3)3CCl + OH-
®
(CH3)3COH + Cl-
The accepted mechanism for the reaction
is
(CH3)3CCl ®
(CH3)3C+ + Cl- (slow)
(CH3)3C+ + OH- ®
(CH3)3COH (fast)
What is a rate law that is consistent with the mechanism for this
reaction? a. | rate =
k[(CH3)3CCl] | b. | rate =
k[(CH3)3CCl][OH-] | c. | rate =
k[(CH3)3C+][OH-] | d. | rate =
k[(CH3)3CCl][OH-]/[Cl-] | e. | rate =
k[(CH3)3CCl] [OH-]/[Cl-] | | |
|
|
| 32. | Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon
dioxide.
NO2(g) + CO(g) ® NO(g) +
CO2(g)
A proposed mechanism for this reaction
is
2 NO2(g) f NO3(g) + NO(g) (fast,
equilibrium)
NO3(g) + CO(g) ® NO2(g) + CO2(g)
(slow)
What is a rate law that is consistent with the
proposed mechanism? a. | rate = k[NO2][CO] | b. | rate =
k[[NO2]2[CO] | c. | rate =
k[[NO2]2[CO]/[NO] | d. | rate =
k[NO3][CO] | e. | rate =
k[NO2]2 | | |
|
|
| 33. | For
the overall reaction
A + 2B ® C
which of the following mechanisms is consistent with the rate equation
below?
rate = k[A] [B]2 a. | 2A + B f
I (fast)
I + B ® C + A (slow) | b. | A + B
® I
(slow)
I + B ® C (fast) | c. | 2B ® I (slow)
A + I
® C
(fast) | d. | 2B f I (fast)
I + A
® C
(slow) | e. | A + 2B ® I (fast)
I + B
® C + B
(slow) | | |
|