Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
| 1. | Which
of the following postulates of Dalton's atomic theory are now known to be
incorrect?
1. | Matter is made up of atoms. | 2. | All atoms of a given element are
identical. | 3. | Atoms are indivisible and
indestructible. | | |
a. | 1
only | b. | 2
only | c. | 3
only | d. | 2 and
3 | e. | 1, 2, and
3 | | |
|
|
| 2. | The
Millikan oil drop experiment determined a. | the mass of a drop of oil. | b. | the charge on an
electron. | c. | the charge to mass ratio of a proton. | d. | the mass of a
neutron. | e. | the number of electrons in a drop of
oil. | | |
|
|
| 3. | Which
of the following particles will not be deflected by charged plates? a. | g particles | b. | b particles | c. | a particles | d. | protons | e. | a and b particles | | |
|
|
| 4. | J. J.
Thomson determined the charge to mass ratio of electrons by a. | creating a
charge on the amber by rubbing it with a cloth. | b. | deflecting
cathode rays with magnetic and electric fields. | c. | exposing
photographic plates to radioactive uranium ores. | d. | striking a
beryllium target with particles emitted from radioactive polonium. | e. | bombarding gold
foil with alpha particles. | | |
|
|
| 5. | From
the results of his gold foil experiment, Ernest Rutherford concluded that a. | electrons have a
charge of -1.602 ´ 10-19 C. | b. | atoms contain
equal numbers of protons and electrons. | c. | uranium ores emit a form of radiation that affect photographic
plates. | d. | alpha particles are helium nuclei. | e. | atoms are
composed of a small, dense nucleus surrounded by a cloud of electrons. | | |
|
|
| 6. | Beta
(b) particles are
identical to ____. a. | neutrons | b. | electrons | c. | helium nuclei | d. | light | e. | protons | | |
|
|
| 7. | Rank
the subatomic particles from lowest to highest mass. a. | electrons =
protons < neutrons | b. | electrons < neutrons < protons | c. | electrons <
protons < neutrons | d. | neutrons < electrons < protons | e. | electrons <
protons = neutrons | | |
|
|
| 8. | An
atomic mass unit (u) is defined as a. | the mass of one hydrogen-1 atom. | b. | 1/8 the mass of
one oxygen-16 atom. | c. | 1/12 the mass of one carbon-12 atom. | d. | 1.99
´
10-23 g. | e. | the sum of the masses of one proton, one neutron, and one
electron. | | |
|
|
| 9. | How
many protons, neutrons, and electrons are in an oxygen-18 atom? a. | 6 protons, 8
neutrons, 4 electron | b. | 6 protons, 10 neutrons, 8 electrons | c. | 8 protons, 8
neutrons, 8 electrons | d. | 8 protons, 10 neutrons, 8 electrons | e. | 8 protons, 10
neutrons, 18 electrons | | |
|
|
| 10. | What
is the atomic symbol for an element with 28 protons and 31 neutrons?
|
|
| 11. | What
is the identity of 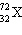 ? ?
|
|
| 12. | Which
of the following atoms contains the largest number of protons? a. | 158Gd | b. | 157Tb | c. | 127I | d. | 138Ba | e. | 144Nd | | |
|
|
| 13. | Which
two of the atoms below have the same number of neutrons?
|
|
| 14. | All
of the following statements are true EXCEPT a. | for any neutral element, the number of protons and electrons
are equal. | b. | electrons and protons have equal mass, but opposite
charges. | c. | the mass number is the sum of the number of protons and
neutrons. | d. | the atomic number equals the number of
protons. | e. | isotopes of an element have identical atomic
numbers. | | |
|
|
| 15. | Which
two of the following atoms are isotopes?
|
|
| 16. | Bromine has two naturally occurring isotopes. The average mass of bromine is 79.904 u.
If 50.54% of bromine is found as bromine-79 (78.9183 u), what is the mass of the other
isotope? a. | 79.82
u | b. | 79.97
u | c. | 80.91
u | d. | 81.93
u | e. | 82.91
u | | |
|
|
| 17. | You
have 0.330 mole of each of the following elements: Be, B, Br, Ba, and Bi. Which sample has the
largest mass?
|
|
| 18. | You
have 4.15 g of each of the following elements: Ca, Cu, Ce, Cs, Cf. Which sample contains the largest
number of atoms?
|
|
| 19. | Calculate the number of moles in 0.41 g titanium. a. | 9.1 ´ 10-4
mol | b. | 8.6 ´ 10-3
mol | c. | 0.051
mol | d. | 2.0 ´ 101
mol | e. | 1.2 ´ 102
mol | | |
|
|
| 20. | What
is the mass of 5.1 mol P? a. | 6.3 ´ 10-3 g | b. | 1.6 ´ 10-1
g | c. | 6.1
g | d. | 1.6 ´ 102
g | e. | 2.0 ´ 102
g | | |
|
|
| 21. | The
molar mass of silver is 107.9 g/mol. What is the mass of a single silver atom? a. | 1.539
´
10-26 g | b. | 1.792 ´ 10-22 g | c. | 1.079
´
10-20 g | d. | 1.901 ´ 10-18 g | e. | 5.581
´ 1021
g | | |
|
|
| 22. | The
density of lithium is 0.546 g/cm3. What volume is occupied by 1.96 ´ 1023
atoms of lithium? a. | 0.0859
cm3 | b. | 0.596 cm3 | c. | 4.14
cm3 | d. | 5.63 cm3 | e. | 39.0
cm3 | | |
|
|
| 23. | Which
three elements are likely to have similar chemical and physical properties? a. | sodium, lithium,
and potassium | b. | sodium, magnesium, and aluminum | c. | nitrogen,
oxygen, and neon | d. | nickel, copper, and zinc | e. | uranium,
plutonium, and americium | | |
|
|
| 24. | Structural isomers are compounds that have a. | the same molar
masses, but different elemental composition. | b. | have identical
structures, but contain different isotopes of the same elements. | c. | two or more
double bonds. | d. | the same elemental composition, but different molar
masses. | e. | the same elemental composition, but the atoms are linked in
different ways. | | |
|
|
| 25. | What
is the general formula for an alkene? a. | CnH2n+2 | b. | CnH2n | c. | CnHn-2 | d. | Cn+2Hn | e. | CnH2n-2 | | |
|
|
| 26. | Which
of the following molecules might be an alkyne? a. | C2H6 | b. | C4H8 | c. | C5H8 | d. | C6H12 | e. | C6H14 | | |
|
|
| 27. | What
is the molecular formula for heptane? a. | C6H12 | b. | C6H14 | c. | C7H14 | d. | C7H16 | e. | C8H14 | | |
|
|
| 28. | What
is the name of the following compound?
a. | 2,5-dimethylheptane | b. | 2,5-diheptane | c. | 2-ethyl-5-methylhexane | d. | 3,6-dimethylheptane | e. | 1,1,4-trimethylhexane | | |
|
|
| 29. | What
is the name of the following compound?
a. | 3,5-diethylmethylheptane | b. | 3-ethyl-5-methylheptane | c. | decane | d. | 2,4-diethylhexane | e. | 3-ethyl-5-methyldecane | | |
|
|
| 30. | What
is the name of the following compound?
a. | 1-methyl-2-pentene | b. | cis-1-methyl-2-pentene | c. | trans-2-hexene | d. | cis-4-hexene | e. | trans-1-methyl-2-propylethylene | | |
|
|
| 31. | What
is the name of the following compound?
a. | trans-acetylene | b. | cis-1,3-pentadiene | c. | trans-2,4-pentadiene | d. | cis-4-methyl-1,3-butadiene | e. | cis-4-methyl-1,3-butene | | |
|
|
| 32. | What
is the molecular formula of this hydrocarbon?
a. | C6 | b. | C6H6 | c. | C6H8 | d. | C6H12 | e. | C6H14 | | |
|
|
| 33. | Chalcopyrite is a copper containing ore. This ore contains 1 iron atom and 2 sulfur
atoms for each copper atom. What is the formula of chalcopyrite? a. | CuFeS | b. | CuFe(2S) | c. | CuFeS2 | d. | 2SCuFe | e. | CuFeS2 | | |
|
|
| 34. | Acetylsalicylic acid, commonly known as aspirin, has 9 carbon atoms, 8 hydrogen atoms,
and 4 oxygen atoms per molecule. What is the molecular formula of aspirin? a. | C9He8O4 | b. | C9H8O4 | c. | C9H8Ox4 | d. | Ca9H8O2 | e. | C9(HO4)2 | | |
|
|
| 35. | A
nitride ion has ____ electrons.
|
|
| 36. | An
aluminum ion has ____ electrons.
|
|
| 37. | Identify the ions present in KHCO3. a. | KHCO3
is not ionic. | b. | KH+, and
CO3- | c. | K+, H+, C4+, and
O2- | d. | KH2+ and
CO32- | e. | K+ and
HCO3- | | |
|
|
| 38. | Which
atom is most likely to form a 1- ion?
|
|
| 39. | Which
atom is most likely to form a 3+ ion?
|
|
| 40. | For a
nonmetal in Group 5A of the periodic table, the most common monatomic ion will have a charge of
____.
|
|
| 41. | Which
of the following formulas is not correct? a. | Al2(SO4)3 | b. | NaClO3 | c. | Ba2O3 | d. | Mg(NO3)2 | e. | KH2PO4 | | |
|
|
| 42. | What
is the correct formula for an ionic compound that contains aluminum ions and carbonate
ions? a. | AlCO3 | b. | Al(CO3)2 | c. | Al(CO3)3 | d. | Al2(CO3)3 | e. | Al3(CO3)2 | | |
|
|
| 43. | What
is the correct formula for an ionic compound that contains magnesium ions and fluoride
ions? a. | Mg2F | b. | MgF | c. | Mg2F2 | d. | MgF2 | e. | Mg2F3 | | |
|
|
| 44. | What
is the charge on the lead ion in PbS2?
|
|
| 45. | What
is the correct formula for sodium acetate? a. | Na2CH3O | b. | NaCH3CO2 | c. | NaCH3O | d. | NaCH2O2 | e. | NaCHO | | |
|
|
| 46. | What
is the correct formula for potassium dihydrogen phosphate? a. | K2H2PO4 | b. | KH2PO4 | c. | KH2PO3 | d. | K2H2P | e. | KH2P | | |
|
|
| 47. | What
is the correct formula for copper(II) chloride? a. | Cu2Cl | b. | CuCl | c. | CuCl2 | d. | CuCl3 | e. | Cu2Cl2 | | |
|
|
| 48. | All
of the following are named correctly EXCEPT a. | LiClO4; lithium
perchlorate. | b. | CaHPO4; calcium hydrogen
phosphide. | c. | NaCN; sodium cyanide. | d. | Mg(OH)2; magnesium hydroxide. | e. | CaSO3; calcium sulfite. | | |
|
|
| 49. | All
of the following are named correctly EXCEPT a. | NiCl2·6H2O; nickel(II) chloride
hexahydrate. | b. | MnO2; manganese(IV) oxide. | c. | TiBr4; titanium(IV) bromide. | d. | AuI; gold(I)
iodide. | e. | FeSO4; iron(I) sulfate. | | |
|
|
| 50. | What
is the correct name for NH4ClO4? a. | ammonia hydrogen
chlorate | b. | ammonia hydrogen perchlorate | c. | ammonium
perchlorate | d. | ammonium hypochlorite | e. | ammonium
hydrochloric acid | | |
|
|
| 51. | What
is the correct name for Cr(NO3)2? a. | chromium(II)
nitrate | b. | chromium(II) dinitrate | c. | chromium
dinitrate | d. | dinitrochromate | e. | chromium(II)
nitride | | |
|
|
| 52. | What
is the correct name for SO2? a. | sulfur oxide | b. | sulfur
dioxide | c. | sulfuric dioxide | d. | sulfuric
acid | e. | sulfurous
oxide | | |
|
|
| 53. | What
is the correct name for PF5? a. | phosphorus pentafluoride | b. | phosphorus
pentafluorine | c. | phosphorus(V) fluoride | d. | phosphorus
fluorate | e. | phosphorus fluorine | | |
|
|
| 54. | What
is the molar mass of cobalt(II) iodide hexahydrate? a. | 212.8
g/mol | b. | 293.9 g/mol | c. | 312.7
g/mol | d. | 420.8 g/mol | e. | 465.1
g/mol | | |
|
|
| 55. | How
many moles are in 4.5 g of AgNO3? a. | 1.3 ´ 10-3 mol | b. | 2.6 ´ 10-2
mol | c. | 0.61
mol | d. | 38
mol | e. | 7.6 ´ 102
mol | | |
|
|
| 56. | How
many oxygen atoms are in 1.50 mol of SO3? a. | 7.71
´ 1021
atoms | b. | 1.12 ´ 1022 atoms | c. | 3.01
´ 1022
atoms | d. | 9.03 ´ 1023 atoms | e. | 2.71
´ 1024
atoms | | |
|
|
| 57. | What
is the mass percent of each element in dichloromethane,
CH2Cl2? a. | 10.06% C, 60.24% H, 29.70% Cl | b. | 20.00% C, 20.00%
H, 60.00% Cl | c. | 24.10% C, 3.11% H, 72.79% Cl | d. | 33.87% C, 0.22%
H, 65.91% Cl | e. | 14.14% C, 2.37% H, 83.48% Cl | | |
|
|
| 58. | Nitrogen and oxygen form an extensive series of oxides with the general formula
NxOy. What is the empirical formula for an oxide that contains 46.68%
nitrogen? a. | N2O | b. | NO | c. | NO2 | d. | N2O3 | e. | N2O5 | | |
|
|
| 59. | Phosphorus, P, is combined with chlorine, Cl2, to give a gaseous compound
with the formula PClx. If you start with 2.097 g of P and isolate 9.298 g of
PClx, what is the value of x?
|